Clinicals and Documentation
Inspection of endoscope instrument channels after reprocessing using a prototype borescope
Adarsh M Thaker, Stephen Kim, Alireza Sedarat, Rabindra R Watson, V Raman Muthusamy
Results: A total of 97 inspections of 59 endoscopes were reviewed. The most common finding was scratches, seen in 51 devices (86%). Channel shredding was found in 35 devices (59%). Intrachannel debris was identified in 22 (23%) of the 97 inspections. No moisture was seen (0%) in the 74 inspections performed after forced-air dry and overnight vertical storage compared with moisture in 5 of 18 inspections (28%) performed after storage alone. No visual evidence of biofilm or simethicone residue was discovered despite its frequent use in our unit.
Sterilization Central: The Value of Borescopes in Detecting Damage, Soil, Fluid, and Foreign Objects in Flexible Endoscopes
Cori L. Ofstead and Krystina M. Hopkins (2020) Sterilization Central: The Value of Borescopes in Detecting Damage, Soil, Fluid, and Foreign Objects in Flexible Endoscopes. Biomedical Instrumentation & Technology: March/April 2020, Vol. 54, No. 2, pp. 146-152.
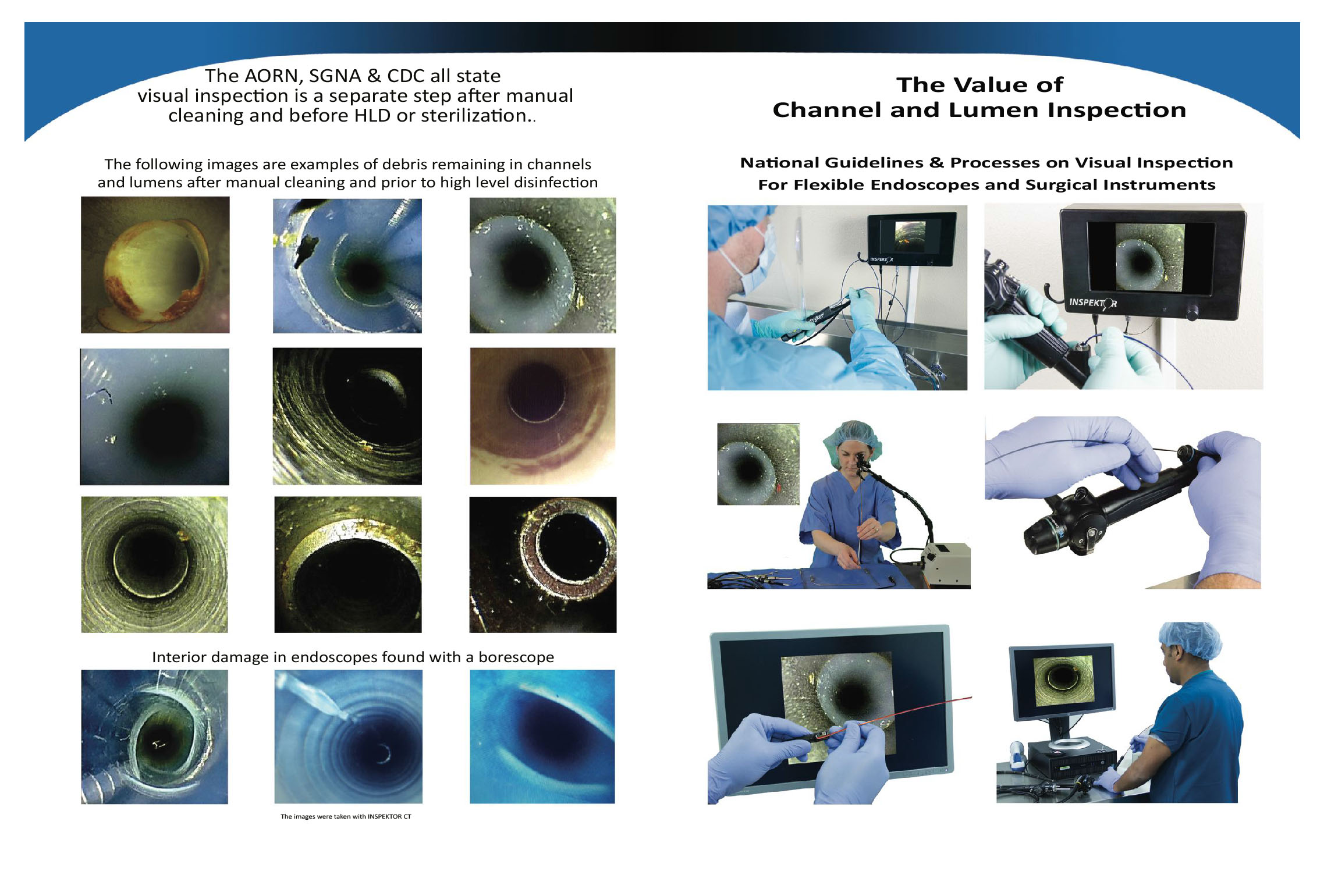
Importance of Visual Inspection During Endoscope Processing
Endoscope manufacturers recommend performing visual inspection every time an endoscope is used, in order to ensure that it is clean and free of damage.1,2 Guideline-issuing bodies, including the Association for the Advancement of Medical Instrumentation, Association of periOperative Registered Nurses, and the Society of Gastroenterology Nurses and Associates recommend using good lighting and magnification for this step.3–5 Visual inspection should be done every time because endoscopes are heavily contaminated during procedures, and organic soil and bioburden (microbes) must be removed prior to high-level disinfection or sterilization for those processes to be effective. In addition, endoscopes are fragile and commonly sustain damage that can harbor contamination during routine use.
The effectiveness of sterilization for flexible ureteroscopes: A real-world study.
Ofstead CL, Heymann OL, Quick MR, Johnson EA, Eiland JE, Wetzler HP.
Am J Infect Control. 2017 Aug 1;45(8):888-895. doi: 10.1016/j.ajic.2017.03.016. Epub 2017 Jun 15.
RESULTS: “Researchers examined 16 ureteroscopes after manual cleaning and sterilization using hydrogen peroxide gas. Every ureteroscope had visible irregularities, such as discoloration, residual fluid, foamy white residue, scratches, or debris in channels.”
Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures.
Ofstead CL, Wetzler HP, Heymann OL, Johnson EA, Eiland JE, Shaw MJ.
Am J Infect Control. 2017 Feb 1;45(2):e26-e33. doi: 10.1016/j.ajic.2016.10.017.
RESULTS: “At final assessment, all endoscopes (N = 20) had visible irregularities. Researchers observed fluid (95%), discoloration, and debris in channels.” …”Eighty-five percent of endoscopes required repair due to findings.”
Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing.
Ofstead CL, Wetzler HP, Johnson EA, Heymann OL, Maust TJ, Shaw MJ.
Am J Infect Control. 2016 Nov 1;44(11):1237-1240. doi: 10.1016/j.ajic.2016.05.016. Epub 2016 Aug 3
RESULTS: Residual fluid was observed inside 19 of 20 endoscopes. Fluid photographed in 8 endoscopes resembled simethicone solutions. FTIR analysis confirmed the presence of simethicone in 2 endoscopes.
Assessing residual contamination and damage inside flexible endoscopes over time.
Ofstead CL, Wetzler HP, Eiland JE, Heymann OL, Held SB, Shaw MJ.
Am J Infect Control. 2016 Dec 1;44(12):1675-1677. doi: 10.1016/j.ajic.2016.06.029. Epub 2016 Sep 7
Abstract: Researchers evaluated flexible endoscope damage and contamination levels at baseline and 2 months later. Postcleaning test results exceeded benchmarks for all gastroscopes and no colonoscopes. Microbial growth was found in samples from 47% of fully reprocessed endoscopes at baseline and 60% at follow-up. Borescope examinations identified scratches, discoloration, debris, and fluid inside endoscopes. Irregularities changed over time. Study evaluations allowed damaged and contaminated endoscopes to be identified and re-reprocessed or sent for repairs.
Outbreak of Pseudomonas aeruginosa Surgical Site Infections after Arthroscopic Procedures
“These SSIs were likely related to surgical instrument contamination with P. aeruginosa during instrument reprocessing. Retained tissue in inflow/outflow cannulae and shaver handpieces could have allowed bacteria to survive sterilization procedures.”
ST79, 7.3 – Manufacturers’ written IFU
The device manufacturer’s current written IFU should be accessible, reviewed, and followed. If there are no specific
written IFU in the labeling, then the manufacturer should be contacted and requested to provide a documented
method of cleaning.
Stryker’s IFU
Recommends a scope an endoscope to inspect their arthroscopic shaver hand piece.
Arthrex’s IFU
Recommends using a scope to inspect their arthroscopic shaver hand piece.
FDA Safety Communications: Ongoing Safety Review of Arthroscopic Shavers. October 6, 2014
“Consider inspecting the inside of the devices (arthroscopic hand pieces) following cleaning to ensure that they have been cleared of any tissue or fluids. There may be multiple ways to accomplish this. As one example, the facility that brought this situation to our attention uses a 3mm video scope to inspect the channels of the shaver hand piece.”
FDA Safety Communications: Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) Duodenoscopes May Impede Effective Cleaning. March 4, 2015
“Thoroughly disinfect duodenoscopes between uses and have in place a comprehensive quality program for reprocessing.”
FDA Safety Communication: Infections Associated with Reprocessed Flexible Bronchoscopes. September 17, 2015
“Implement a comprehensive reprocessing quality control program. Your reprocessing program should include written procedures for monitoring, training and adherence to the program, and documentation of equipment tests, processes, and quality monitors used during the reprocessing procedure.”
CDC Health Advisory – Immediate Need for Healthcare Facilities to Review Procedures for Cleaning, Disinfecting, and Sterilizing Reusable Medical Devices – September 15, 2015
“Recent media reports describe instances of patients being notified that they may be at increased risk for infection due to lapses in basic cleaning, disinfection, and sterilization of medical devices. These events involved failures to follow manufacturers’ reprocessing instructions for critical and semi-critical items and highlight the need for healthcare facilities to review policies and procedures that protect patients.”
ST79, 7.6.4.5 – Verification of the cleaning process
“After completing the cleaning process, personnel should visually inspect each item carefully to detect any visible soil.”
The Value of Lumen Inspection, Communique’,
May/June 2015, page 76 – 78
“With today’s lumen inspection systems, departments can now visually confirm that the insides of instruments are as clean as the outside and improve the standard of care.”
Visual Inspection of Flexible Endoscope Working Channels Communique May-June 2016
Analysis found 100% of 494 suction tubes and 15 arthroscopic hand pieces endoscopically inspected had foreign matter in them.